Drug Formulations & Precursors:
Simple, Low-Cost
All drug formulations have a unique fingerprint of both active and inactive ingredients that identifies the efficacy and brand of each drug. Incorrect formulations containing foreign or substitute ingredients can jeopardize a patient's well-being. Additionally, when incorrect or counterfeit formulations are distributed under a brand name, the reputation of the manufacturer is at risk. Drug manufacturers must protect their business interests by ensuring that the correct formulations of their products are being distributed. This will ensure that patients are receiving the correct formulation, that their branding isn't compromised by counterfeiters, and that they have documented fingerprint records of all their drug and formulation steps. A non-destructive fingerprinting method for drug formulations is essential for pharmaceutical manufacturers. It offers factual support for patent and other legal records as well as retention of the original powder material if needed. |  |
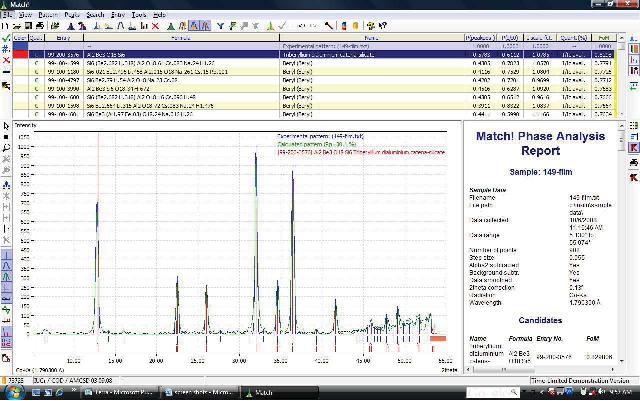
A drug formulation, at any stage, can be quickly scanned for its fingerprint by measuring its crystalline structure. Pharmaceutical fingerprinting is commonly done using XRD. The BTX combines both XRD fingerprinting and XRF elemental ID to perform rapid, thorough identification with its unique sample handling system that leaves the formulation intact for further or future testing.
Operation of the BTX is as EASY as 1, 2, 3 | |
 | 1. Place powdered drug into holder 2. Insert sample holder into BTX 3. Press "Start" to record fingerprint |
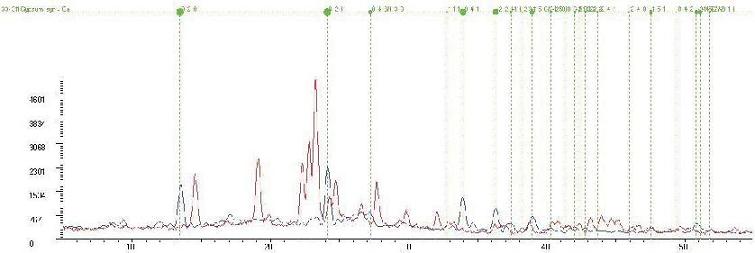
Popular Male Enhancement Drug Formulation Fingerprints from Two Different Distribution Channels
The red fingerprint is the manufacturer's correct formulation of the male enhancement drug. The blue fingerprint is marketed as the same male enhancement drug and distributed on the internet. It is actually gypsum and contains no active ingredient for the male enhancement drug at all.
Available phase identification pattern matching software provides a complete analysis using either publicly available diffraction pattern databases or common commercially available databases, such as PDF2/PDF4.
| Basic Specifications | |
| Weight: | 14.5 kg with 4 batteries |
| Size: | 48.5 x 39.2 x 19.2 cm / 19.1 x 15.4 x 7.6 in |
| XRD Resolution: | 0.25° 2T FWHM |
| XRD Range: | 5-55° 2T |
| Detector Type: | 1024 x 256 pixels; 2D Peltier-cooled CCD |
| XRF Energy Resolution: | 230 eV (at 5.9 keV) |
| XRF Energy Range: | 3-25 keV |
| Sample Grain Size: | <150µ crushed minerals (100 mesh screen, 150 um) |
| Sample Quantity: | >15mg; smaller sample holder available on special order |
| X-Ray Target Material: | Cobalt (others available on request) |
| X-Ray Tube Voltage: | 30kV |
| X-Ray Tube Wattage: | 10W |
| Field Autonomy: | ~4 hours (can be expanded by hot swapping batteries) |
| Power Consumption: | 85-90W during analysis |
| Data Storage: | 40Gb; Ruggedized internal hard drive |
| Wireless Connectivity: | 802.11 b/g for remote control from web browser |
| Operating Temperature: | -20° to 35°C |
| Enclosure: | IP67, MIL C-4150J ; rugged case |
| Specifications subject to change without notice. | |