In a recent webinar hosted by Simon Nordstad, CEO and Founder of myStandards GmbH, participants were treated to a comprehensive overview of analytical techniques, sample preparation strategies, the importance of reference materials, and essential QA/QC concepts relevant to geochemistry and alloy analysis. Here’s a recap of the key insights shared during this engaging session.
Understanding the Analytical Techniques
Comparison of four key analytical methods:
- ICP-MS (Inductively Coupled Plasma Mass Spectrometry)
- ICP-OES (Optical Emission Spectroscopy)
- LIBS (Laser-Induced Breakdown Spectroscopy)
- XRF (X-ray Fluorescence)
No single technique is universally superior—each serves different analytical needs depending on the elements of interest and required sensitivity.
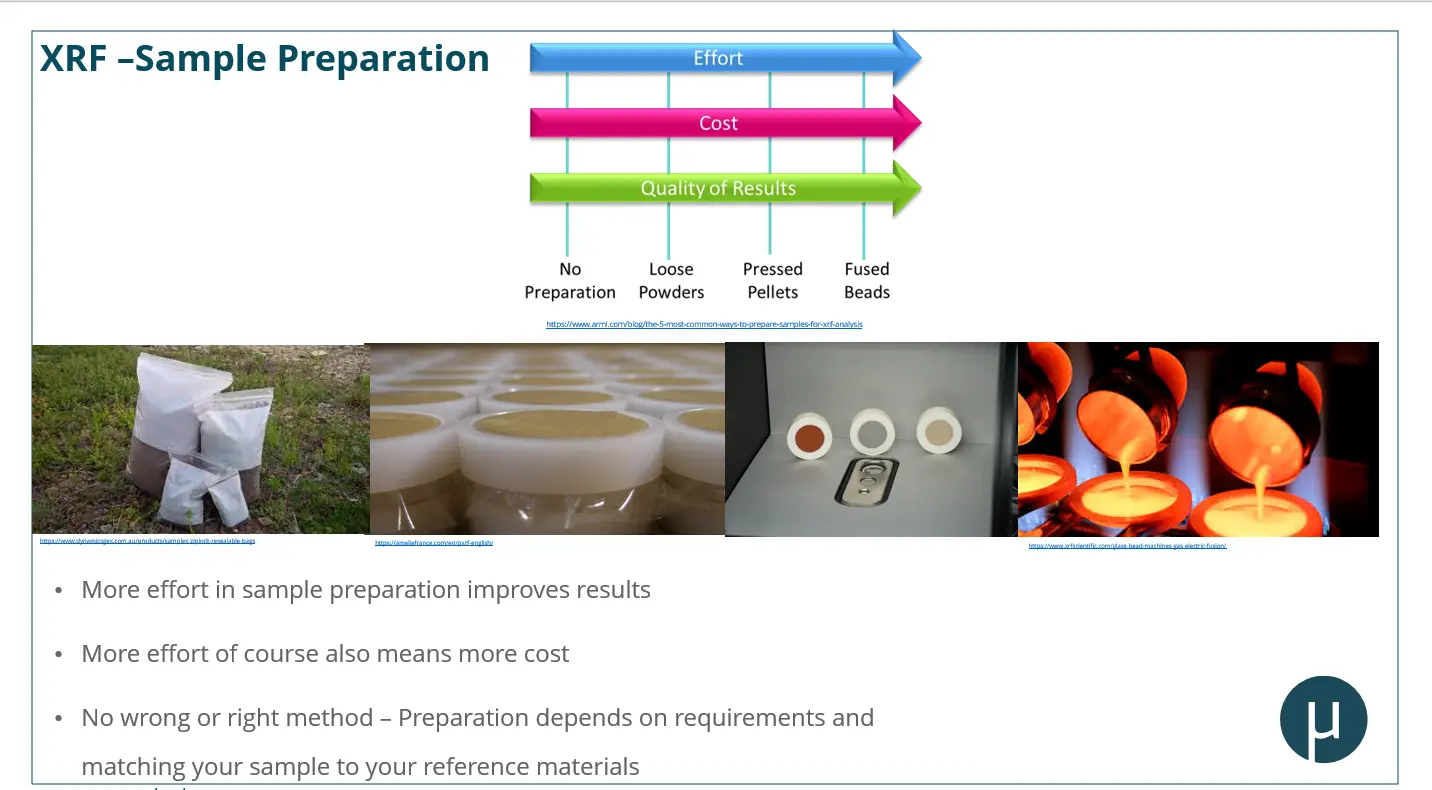
Sample Preparation: Why It Matters
Sample preparation plays a critical role in achieving accurate and reliable results—especially for XRF. The webinar outlined common prep methods:
- Loose powders in sample cups or bags
- Pressed pellets, with or without binders
- Fused beads, typically for lab-based analysis
- Flat metal surfaces for alloy testing
While minimal prep can suffice for handheld XRF, investing in better preparation—like using pressed pellets—can significantly improve data quality.

Vanta Max XRF analyzer analyzes a soil sample in the field.
Reference Materials and Calibration
The session also covered the distinctions between Reference Materials (RMs) and Certified Reference Materials (CRMs). According to ISO standards, CRMs undergo rigorous validation procedures and come with a certificate indicating value, uncertainty, and traceability.
Advice is that the best way to ensure analytical confidence is to use materials that meet ISO 17034 accreditation or at least follow its standards transparently.
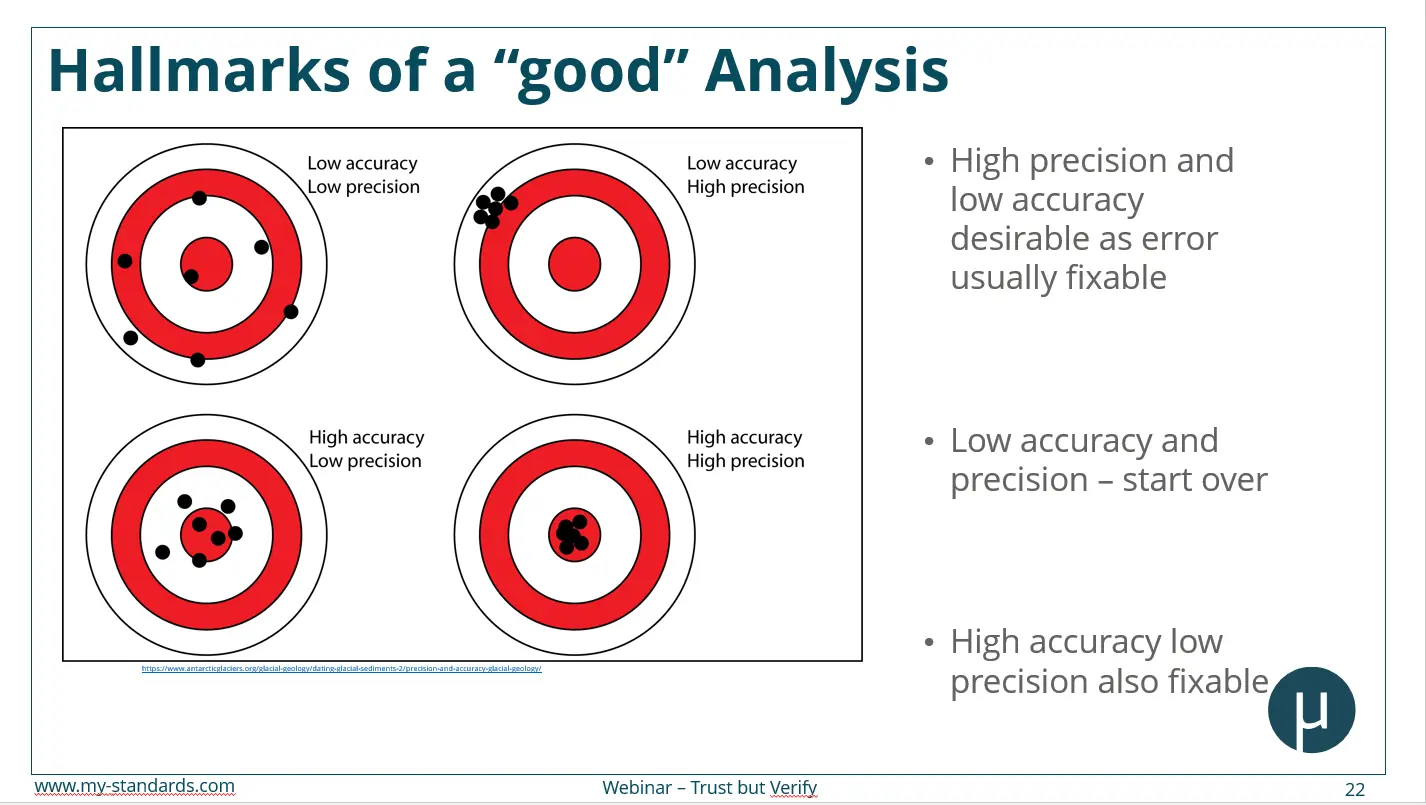
QA/QC Concepts: Accuracy and Precision
There are two cornerstone concepts of quality assurance:
- Accuracy: How close a result is to the true value.
- Precision: How consistent repeated measurements are.
How XRF calibration works? By plotting the counts-per-second data from the XRF spectra against known concentrations from reference materials, analysts can build reliable regression models.
The Matrix Effect: Why Calibration Must Match Your Material
Not all samples behave the same during analysis. The “matrix effect” refers to how the overall composition of a sample (its matrix) can influence the analytical results—even when the elemental concentration remains the same.
The solution? Matrix-matched calibrations. Your calibration should be based on samples of the same type as you intend to measure.
Sample Prep Matters—Even for the Same Sample
Sample preparation directly affects the output of XRF analysis. Consistency is key: your samples must be prepped in the same way as your calibration standards to maintain accuracy.
Quality Assurance vs. Quality Control: A Quick Breakdown
There is a clear distinction between QA and QC:
- QA (Quality Assurance): A proactive, process-based strategy to prevent defects. It sets your quality goals.
- QC (Quality Control): A reactive, product-based method to detect and correct errors. It verifies your results.
- In XRF, this means ensuring your device consistently provides accurate results by monitoring those results over time.
Field-Proven QA/QC Practices
To ensure long-term precision you should monitor performance of your instrument. A few methods for this are:
- Sample bracketing: Insert known standards between sets of sample measurements.
- Cross-validation: Compare results from XRF with other techniques like ICP-MS.
- Repeatability checks: For example, measuring the same pressed pellet 100 times showed less than 1% relative deviation—an excellent indication of precision.

Monitor instrument performance in the field with the Vanta Max XRF analyzer.
Defining Control Limits
Evident’s handheld XRF instruments help users verify results in real-time. For example, alloy verification specifications for many alloys are built into the analyzer, comparing elemental ranges to spec automatically. Additionally, by running repeat tests on a single sample, users can determine their instrument’s inherent precision and set custom control limits.
Trust the Machine—But Always Verify
A handheld XRF analyzer is only as accurate as its calibration and usage allow. It’s not a “point-and-shoot” solution—matching samples to the correct calibration and applying strong QA/QC practices is essential. Otherwise, precise-looking results may still lead to costly mistakes.
“You can trust the machine to give you the same number repeatedly—but whether that number is right? That’s on you.”
Want to learn more? Watch the full webinar replay to explore all the tools and techniques discussed and gain confidence in your XRF results—no matter the matrix.
Whether you're working in mining, metallurgy, or material science, this webinar reinforced the importance of choosing the right technique, preparing your samples with care, and validating your results with trustworthy reference materials.
If you missed the live session, be sure to tune in for a deeper dive into the science behind the methods and best practices that drive analytical confidence in solid-state geochemical and alloy analysis.
Related Content
XRF Acronyms and Abbreviations Decoded: Quick Reference Guide

.jpg?rev=AD55)